Leading Pharmaceutical Company in Egypt: EIPICO
EIPICO (Egyptian International Pharmaceutical Industry Company) is the leading pharmaceutical company in Egypt.

Profile: About EIPICO
Egyptian International Pharmaceutical Industries (EIPICO) is the leading pharmaceutical company and, at the same time, the largest local producer of pharmaceuticals in Egypt.
The pharmaceutical giant exports their pharmaceutical products to Arab Countries, and to some African, Asian and East European Countries.
The company is clearly the industry leader, capturing 8% share of the local pharmaceutical market and approximately 20% of Egypt’s total drug exports.
EIPICO is a public company, listed on Egyptian Exchange since September 1995. It operates within the pharmaceuticals, biotechnology & life sciences sector focusing on pharmaceuticals.

Address: First Industrial Zone, 10th of Ramadan City, Egypt, P.O. BOX 149
Website: www.eipico.com.eg
Interview: EIPICO’s Dr. Borhan El-Din Ismail: “I myself raised the pharmaceutical industry in the Middle East”
“I myself raised the pharmaceutical industry in the Middle East. I started very young – I was 32 and I was a chairman of a government company and I started the pharmaceutical industry here in Egypt in 1962, it was nothing at all.”
History of EIPICO
EIPICO was established in December 1980 and started production in 1985. It currently has 3 subsidiaries operating across Egypt. The company is based in 10th of Ramadan, Egypt.
The three EIPICO subsidiaries are: Egyptian International Company for Ampoules (98.63%), EIPICO Tech (98.60%) and Saudi Universal Factory (30%).
Today, EIPICO produces more than 300 products covering 23 therapeutic groups including all known pharmaceutical forms either traditional or non-traditional dosage forms such as the soft gelatin capsules, spansules, lyophilized products, gels, sprayers and effervescent tablets.
In addition, EIPICO was the first pharmaceutical company in Egypt to produce dosage forms like the spansule capsules (long-acting).
Moreover, the company inaugurated its Biotechnology Center on August 6th, 2001 to contribute in the development of the drugs in all stages such as:
– Synthesis of raw materials using biotechnology;
– Bacterial and tissue utilization to produce Biotechnology and pharmaceutical products;
– Extraction of active materials from natural products and others.
License Agreements
EIPICO made license agreements with a group of international pharmaceutical companies, including PFIZER, BOEHRINGER INGELHEIM and many others, to produce their products locally to replace the imported ones and cover the increasing demand and to acquire technology and know-how.

EIPICO’s Products
EIPICO produces more than 300 products covering 23 therapeutic groups including all known pharmaceutical forms either traditional or non-traditional dosage forms such as the soft gelatin capsules, spansules, lyophilized products, gels, sprayers and effervescent tablets.
EIPICO products have been selected according to the actual needs taking into consideration the following priorities:
• New products to replace the imported ones.
• The new technology in pharmaceutical industry and researches.
• The mutual co-operation between the Egyptian experts and researchers at EIPICO and those of the foreign international pharmaceutical companies according to license agreements.
Main Products:
Alimentary Tract
Blood Forming Organs
Cardiovascular Drugs
Genito-Urinary System
Dermatologicals
Systemic Hormones
General Anti-Infectives
Musculoskeletal System Drugs
Acting Drugs
Antiparasitics
Pulmonics & Antihistaminics
Sensory Organs (Ophthalmologicals)
Ophthalmics

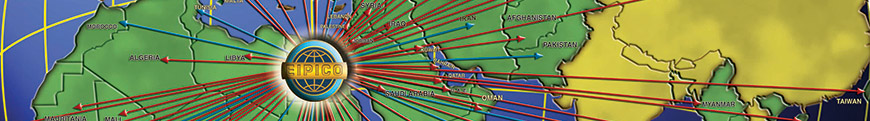
EIPICO’s Exports
Dr. A. Borhan El-Din Ismail, Chairman and Executive Director of EIPICO, explains:
“In 1995, I was number one in the Middle East and because of that I have to go abroad to prove that I am number one here and there. My name in the Middle East is very well known.”
“As we started big we have to keep going and in order to increase consumption and to progress we should export. As I told you, we have to import a lot of things for our use. I have to cover this cost with exports. If I succeed, I think I will reach the maximum that I want because my target is different to the targets of the multinationals. The multinationals want to expand but my main reason for exporting is to get foreign currency to address my need for foreign currency. When I reach my target for the local market as achieved by exporting, I will have reached the best position I want to put my company in.”
EIPICO exports their produce to:
I am increasing my power in every place I’m in. For example, in England we started with 1 product, now we have 3 products. My aim is to increase my products where I am now, and expand into other countries every year in order to reach my target.
Middle East:
Bahrain
Iraq
Jordan
Kuwait
Lebanon
Oman
Palestine
Qatar
Saudi Arabia
United Arab Emirates
Yemen
Africa:
Algeria
Benin
Eritrea
Ethiopia
Ghana
Kenya
Libya
Mali
Namibia
Somalia
Sudan
Tanzania
Uganda
Europe:
Albania
Belarus
Ireland
Moldova
Romania
Ukraine
United Kingdom
Asia:
Azerbaijan
Georgia
Kazakhstan
Kyrgyzstan
Russia
Tajikistan
Turkmenistan
Uzbekistan

EIPICO & Quality Policy
QMS
EIPICO’s Q.M.S. meets the requirements of both: cGMP regulations and ISO 9001, issue 2000 Standards.
It aims to ensure that the Quality of EIPICO’s products are:
• Conforming to the updated specific requirements.
• Safe.
• Effective.
EIPICO’s Q.M.S. is the way to keep our Quality up. This goal is achieved only by building the Quality into the product during:
• Planning of the Infrastructure needed to achieve conforming to product requirements.
• Planning to improve competence, awareness and training to EIPICO’s human resources.
• Customer communication.
• Research and development.
• Raw materials, packaging materials and other specific materials purchasing process.
• Updating the technology and manufacturing techniques.
It is not only documented and implemented but also its effectiveness is continuously measured, analysed and improved through monitoring and measuring of:
• Customer satisfaction.
• Internal audits.
Moreover, EIPICO’s Q.M.S. promotes the adoption of a “PROCESS APPROACH” when developing, implementing and improving the effectiveness of a Q.M.S., to enhance customer satisfaction by meeting customer requirements.
 Awards & Certificates
Awards & Certificates
In 1996, EIPICO has been awarded the ISO – 9001 Certificate by the (BSI) for establishing, Implementing, and maintaining quality assurance systems in Design, Development, Production, Installation, and Servicing.
In 1999, EIPICO has also been awarded the ISO – 14001 Certificate regarding Environmental – friendly practices.
Premises & Equipment
EIPICO typically applies c.G.M.P. Regulations throughout the operational departments including evaluation of raw materials, calibration of equipments, control, holding and distribution of finished products.
EIPICO plant has a U-shaped design that minimizes mix-up and/or contamination by providing a single direction of material flow and is also surrounded by a green area as a protection from surrounding environment. Each pharmaceutical process has enough space to be carried out apart to minimize mix-up and/or cross contamination.
EIPICO is unique by its Sterile Areas (which are the largest in the Middle East and North African Region) that are environmentally controlled to obtain filtered air up to 97% – 99.7% particulate-free. The water purification system in EIPICO was designed to supply the production zones with dematerialized and distilled water.
Environmental Policy
Realizing the Importance of environmental protection for safe environment for the present and future generations, EIPICO is committed to:
• Providing safe and effective pharmaceutical products according to GMP with continual reduction of impacts.
• Compliance with legal regulations and other requirements.
• Prevention of pollution and conservation of resources.
• Continuous enhancement of the performance, competence and awareness of EIPICO’s employees.
Therefore, EIPICO has established, implemented and maintained integrated management systems for Quality Environment satisfying the requirements of both ISO 9001 and ISO 14001.