Cutting-edge Medical Imaging Equipment for the Diagnosis of Breast Cancer – from CapeRay
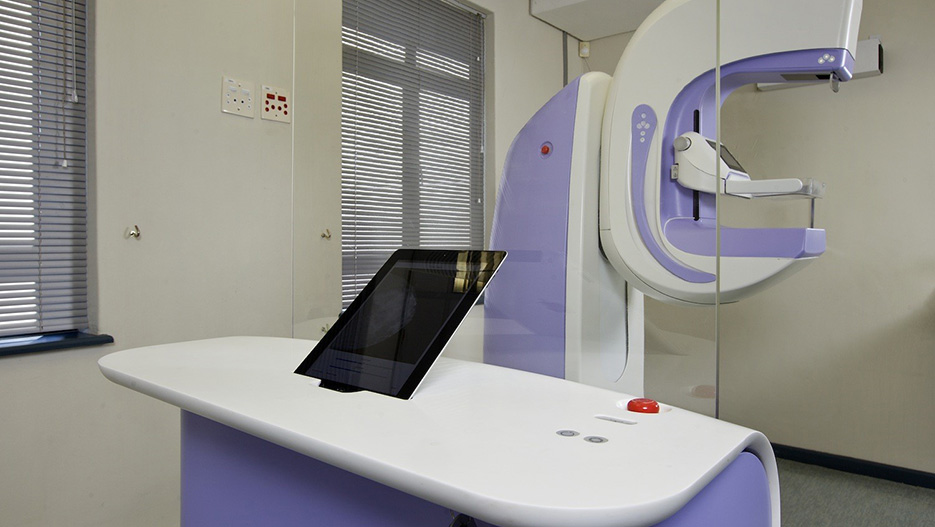
A revolution in breast imaging. An innovative dual-modality imaging system that integrates full-field digital mammography and automated breast ultrasound in a single platform.
Interview with Dr Kit Vaughan, CEO of CapeRay

CapeRay is bringing three mammography devices to market; can you start by giving us a quick overview of CapeRay and its products?
CapeRay is a spin out company from the University of Cape Town. It spun out 6 years ago. The focus of the company is the detection of breast cancer; we have developed imaging products that are specifically geared towards women who have dense breast tissue. The three products that you referred to are: one product which is x-ray only, the second is both x-ray and ultrasound and the third product which has not yet been developed combines three dimensional x-ray with three dimensional ultrasound.
The PantoScanner which combines low dose digital x-rays with ultrasound has been your main product. What was so revolutionary about this technology?
The revolutionary part is the ability to combine the two technologies. Traditional mammography relies on x-rays and that has been around for forty or fifty years, while ultrasound has also been around for forty or fifty years. What is revolutionary is the ability to combine the two separate imaging technologies into a single product and a single platform. Each of them sees different things in terms of what is there within the breast. It turns out that x-ray has very fine spatial resolution but does a poor job when the woman has dense breast tissue. On the other hand, ultrasound tends to be a much coarser imaging modality, but does well with dense tissue and so it can detect the cancers that are missed by x-ray.
Traditional mammography relies on x-rays and that has been around for forty or fifty years, while ultrasound has also been around for forty or fifty years. What is revolutionary is the ability to combine the two separate imaging technologies into a single product and a single platform.
Thus these two in conjunction are basically mammography squared?
I think that would be a fair way to describe it. It makes the job of the person who is making the diagnosis, the radiologist, that much easier because they have got both technologies and both sets of images to look at. With that better job, it is going to be good news for the women as well because there will be a lower possibility of a missed cancer which is the risk that mammography currently presents.
And it should increase the prevention and detection rates.
We believe it will increase the detection rate and the literature supports that. Of course with the earlier detection it means women can be treated at an earlier stage and therefore the chances of a successful outcome of that treatment will be significantly enhanced.
The company is focusing on Europe first as its primary market, but where else will you hope to receive regulatory approval for the product?
I think it would be fair to say that our primary market has to be our local market which is South Africa. There is a demand for such technology here and we are working with various institutions including the National Department of Health to encourage them to consider setting up a screening program. However, the requirement from a regulatory point of view means that we need to secure the CE mark, the European stamp of approval, to sell our product in South Africa. We are working towards that CE mark which we anticipate being able to secure within the next 6 months – by July 2016. That will allow us to look after our primary market which is South Africa but it will also allow us to start selling the machine into Europe as well.
Does South Africa not have its own regulatory system for these?
We have a regulatory system that is called the Medicine Control Council and it has encapsulated medical devices as well but what it doesn’t have is the ability to do its own audits of the various medical technologies. In a sense, what the South African authorities require is that we show equivalence and therefore from an auditing point of view, if we are acceptable in Europe, then we will pass muster here in South Africa.
At that stage when you have a CE approval in 6 months you will initially focus on your domestic markets then regionally and then hopefully Western Europe?
Yes but those can be fast tracked and can be done in parallel so I would anticipate that in the latter part of this year, 2016, we will be exhibiting at international conferences with a view to selling our systems internationally as well.
So you are literally reaching the tipping point with the technology.
Correct. We are in that final, crucial stage of getting the regulatory approval and part of the tests for this is currently on going.
Now for more of a macro question—you have helped to pioneer the medical device industry in South Africa, how do you think South Africa and the Western Cape in particular can enhance its position in the medical world as a medical equipment manufacturer?
I think we have done well in the Western Cape. I write a weekly blog about various activities in my field including more generally about medical devices and I have compared the Western Cape as a medical device destination with other areas in the world such as the United States where there are places in Minnesota and Indiana with medical device companies, and there is also another hub in Boston. In the Western Cape we have about 20 companies that manufacture devices according to European Union standards and they are doing all of their manufacturing here, making products for the local market and for the international market so I believe the Western Cape is certainly onto something very positive.
So it is quite a hot house here?
It is a hot house. It is a crucible for new product development and for high quality products across a range of areas.
How would you characterise the opportunities for local companies to manufacture medical devices? How difficult is it to sell these into South African hospitals? Can they even afford them? What are the challenges to achieve this?
The opportunities are significant but there are some challenges, not the least of which are the lack of accredited companies to perform independent testing for electrical safety and electro-magnetic compatibility. This means our products must be sent overseas for testing which is both expensive and time-consuming. With the CE mark, we can sell our products into South African hospitals although there is no preference given to local manufacturers. Our locally made products have to be able to compete on both price and quality. There is sometimes the perception among both hospitals and doctors that a locally manufactured product cannot be as good as the equivalent imported product.
Is there even a comprehensive mammography screening program in South Africa?
There is not. So the screening that is done is done primarily in the private sector but that is a small part of what goes on in the broad spectrum of healthcare in South Africa. There is a challenge for us as a company manufacturing this product to provide the evidence and to make the case to the National Health Authorities that in fact it is not just about infectious diseases, that non-communicable diseases such as cancer and in particular breast cancer are a major challenge. To treat a woman with advanced stage breast cancer is extraordinarily expensive and the chances of outcome are very low, whereas by screening and detecting that cancer early, you have two benefits: first, it is much less expensive to treat her; and second, her chances of success are significantly improved.
So it is a false economy in a sense. In the health system in this country a lot of the focus is on the communicable diseases for obvious reasons. Is it seen as something of a luxury let’s say?
I think that would be fair to say. I think there is a feeling that we need to put our resources into the most immediate things but the reality is there are many women being diagnosed with breast cancer that could have been treated earlier and we would have had a good chance of successful outcome. You are right, there are some competing interests. To come back to your question about whether the country and the government hospitals can afford a product like ours: it is not going to be cheap: 250,000 to 300,000 dollars in South African rand is a lot of money, about 5 million rand, but the alternative is to buy an expensive imported product or as we would say, two products, an x-ray machine and an ultrasound machine. We have done the numbers; we have shown how by detecting cancer earlier and treating those women who would have been missed and who are false negatives, you can in fact save significant amounts of money. By catching just a few thousand women, you can save millions of rand. We have also done the analysis for the United Kingdom, for their National Health Service, and have demonstrated that our systems could be paid for quite easily by cost savings through earlier treatment.
We understand that currently prototype medical devices need to be sent overseas for testing, how would you develop a better infrastructure that supports local companies and help them to develop the market?
Well I think you have put your finger on a major problem faced by medical device companies in South Africa. Not only is my company looking to raise finances and significant capital to support the company, when we do need to get a product to market, we need to get that international stamp of approval and that is provided by companies which are typically based either in Europe or elsewhere in the world. These companies either fly out here to do their testing or we have to transport our product to their test site overseas. If we had that infrastructure in the country where there were companies, perhaps a subsidiary of an international company, a significant part of our costs in getting a product to market would be reduced. That in itself would be a major saving for companies like mine.
What are some of the strong competitive advantages that South Africa has in the medical technology space?
There are people with good ideas; here in the Western Cape we have four institutions of higher learning, each of which has an engineering faculty and two of them have medical faculties and so in the medical device space there is every opportunity for new products to be developed. We have two government entities which are the Department of Trade and Industry and the Department of Science and Technology which are very supportive of new product development and in fact have programs from which we have benefited. For example, when we take out an international patent, half the costs will be borne by the Department of Trade and Industry. When we go to an overseas conference we get something of the order of 70,000 or 80,000 rand, which may not sound like a lot in US dollars but it certainly is a big boost for us. If we bring in overseas investors, here again we get matching funding from the government for that. I think we have got some good programs in place that assist companies like mine wanting to develop a new product, in our case, a medical device.
Lastly, we want to ask you about the proposed National Health Insurance scheme, NHI. The sceptics have pointed out the very substantial cost attached to it and the administrative complexity that it would involve. It is something that has been a bit of a pie in the sky proposal? Do you foresee it ever becoming a reality?
I think the government’s biggest challenge is going to be funding it adequately. Do we have the tax base in this country to fund such a program? Of course, if we look to models elsewhere in the world such as the United Kingdom with the NHS, and we ask ourselves, do we have the necessary funding to support such a program? That I think is going to be the government’s biggest challenge. There is no two ways about it, if we had a successful national health insurance program it would be great but what people worry about is what it will do to the private healthcare system. Is it going to undermine that in some way? But that is perhaps a selfish attitude by people who can afford private health care. Ultimately there are lots of competing interests as you well know. Healthcare is not the only demand that people are making, there is education and so on. I think NHI is a good idea in principle but in practice we shall have to wait and see. Of course one thing that the Department of Health is trying to do is to pick a few pilot programs to try and run it out to see whether it proves to be successful or not. I am taking a wait and see approach.
FAIR USE POLICY
This material (including media content) may not be published, broadcasted, rewritten, or redistributed. However, linking directly to the page (including the source, i.e. Marcopolis.net) is permitted and encouraged.